COVID-19 Antigen Rapid Test Kit JOYSBIO
15 Individually Wrapped Tests- Prevent Cross infection
In June 2020, JOYSBIO Biotechnology proudly released a new COVID-19
Antigen Rapid Test Kit (Colloidal Gold). The new coronavirus
antigen test kit is a lateral flow immunoassay for the qualitative
detection of SARS-COV-2 antigen (nucleocapsid protein) in upper
respiratory Samples with nasal swabs or saliva during the acute
phase of infection.
Features
- 15-minute rapid detection
- Easy-to-operate coronavirus antigen test
- Less-invasive nasal (NS) swab sample collection
- CE-IVD marked
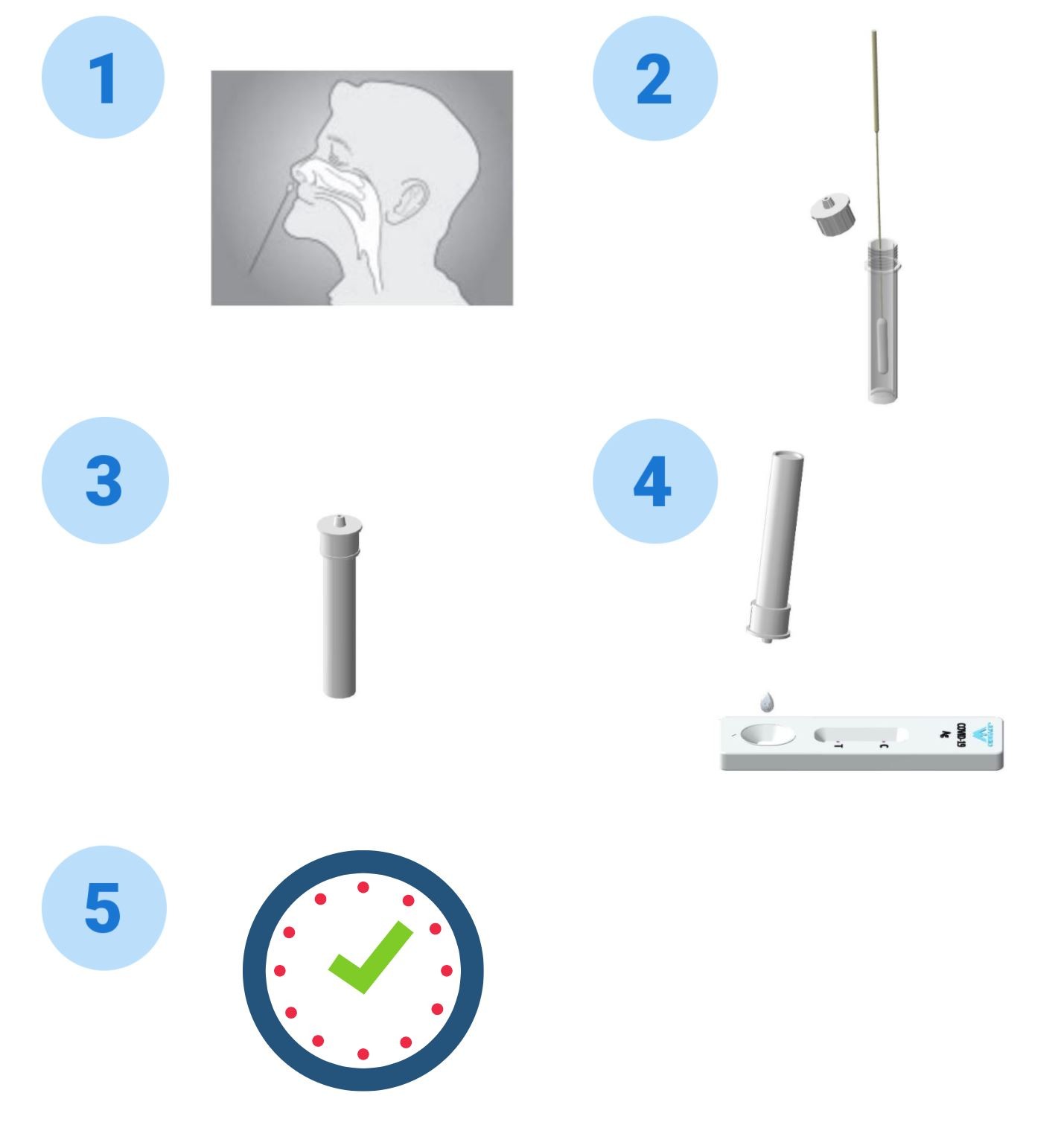
COVID-19 Antigen Test Procedure
- Twist off the cap of the buffer bottle, carefully dispense all buffer into the extraction tube.
- After collecting upper respiratory sample with nasal swab, insert
the swab into the extraction tube, plunge the swab up and down in
the fluid for a minimum of 10 seconds. Hold the swab against the
bottom of the tube, rotate three turns. DO NOT splash liquid out
of the tube.
- Remove the swab while squeezing the sides of the tube to extract the liquid from the swab.
- Press the nozzle cap firmly onto the extraction tube. Mix thoroughly by swirling or flicking the bottom of the tube
- Gently squeeze the tube’s rigid body, dispense two (2) drops of the buffer-specimen mixture into the sample well on the coronavirus antigen test cassette.
- Read the test results between 15 and 20 minutes. Do not read the results after 20 minutes.
Please check Instructions for Use for complete procedure
JOYSBIO
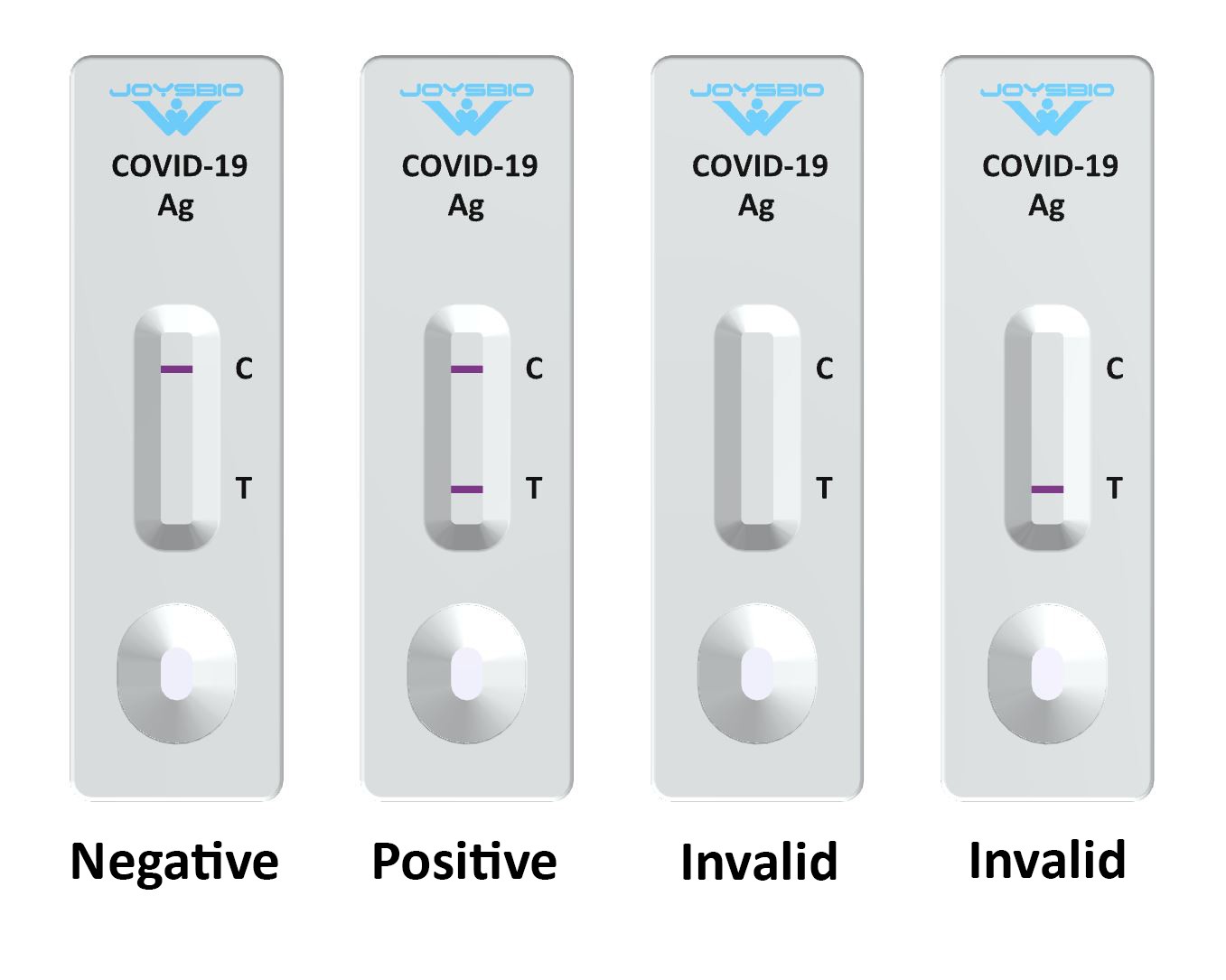
Interpretation of Test Results
- NEGATIVE: A colored band appears on the control line (C line); no colored band shows up on the test line (T line). A negative result indicates there is no coronavirus antigen (N protein) in the specimen, or the level of coronavirus antigen is below the detection limit.
- POSITIVE: A colored band appears on the control line (C line), a second colored band shows up on the test line (T line). A positive result indicates the presence of COVID-19 antigen (N protein) in the patient sample.
- INVALID: No colored band appears on the control line (C line). An invalid test result suggests there might be insufficient buffer volume or incorrect operating procedures. Carefully review the test procedure and test the same patient again with another coronavirus antigen rapid test cassette. Contact your distributor if the problem persists.
Performance Characteristics
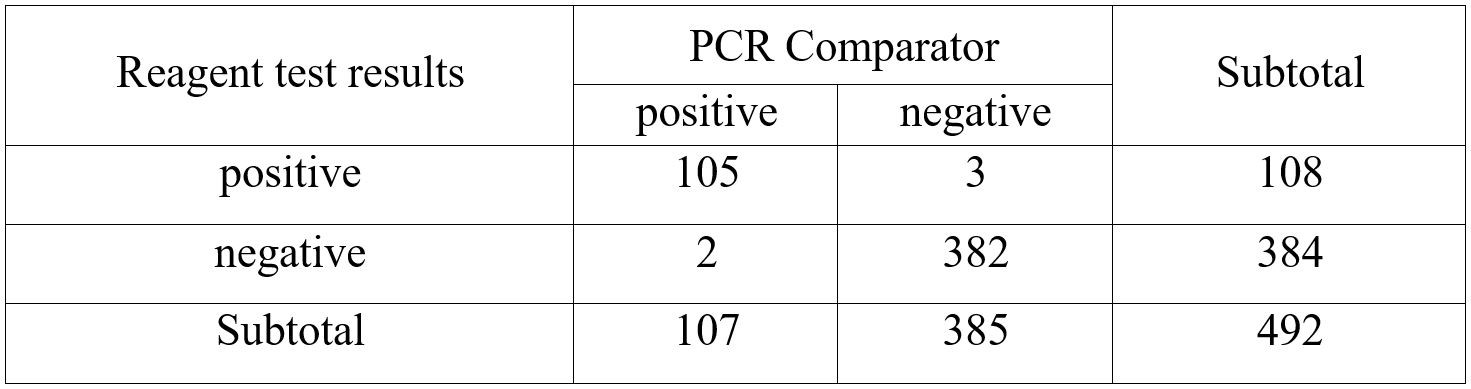
JOYSBIO’s coronavirus Ag test kit was independently evaluated at Centro Diagnostico Delta S.r.l. in Italy between October 2020 and January 2021. A total of 107 positive specimens were tested with JOYSBIO’s COVID-19 Antigen Rapid Test Kit. These specimens were collected from patients who are suspected of COVID-19 with nasal swabs. The coronavirus antigen test kit’s sensitivity and specificity are compared against a CE-IVD marked RT-PCR test kit. This clinical evaluation is conducted under the assumption that SARS-CoV is no longer spreading in the community. According to the clinical analysis of 492 samples, the detection sensitivity is 98.13%, and the specificity is 99.22%.
- Positive Percent Agreement (PPA) = 105/107 (98.13%) (95%CI: 93.4%~99.8%)
- Negative Percent Agreement (NPA) = 382/385 (99.22%) (95%CI:97.7%~99.8%)
- Accuracy = (105+382)/492×100%=98.98%
- Kappa = 2×(105×382-3×2)/(108×385+107 ×384) = 0.97>0.5
The limit of detection (LOD) of this product is 1.6 x 102 TCID50/mL, calculated through a gradient dilution method.
COVID-19 Antigen Rapid Test Kit JOYSBIO Instructions 
COVID-19 Antigen Rapid Test Kit JOYSBIO- Brochure 
Pack Size: Single Test (15 Individually Wrapped Tests per Box)
Coronavirus Antigen (Ag) Rapid Test Kit Principle
The conjugation pad also contains colloidal gold-labeled chicken IgY, which is captured by Goat anti-chicken IgY on the control line as procedural control. A colored band on the control line represents the proper liquid flow through the cassette; the absence of a colored band on the control line indicates insufficient sample or buffer volume.